Hybrid Crystallization of Organic Chemicals
Separation of the various mixtures can be achieved using the different physical properties of various components. Distillation is often used to take advantage of the difference in relative volatility of the target component versus impurities. This often works well, but if the relative volatility of isomers is close it becomes impossible to separate them via distillation. Other considerations with distillation include high energy costs and its negative impact on product quality.
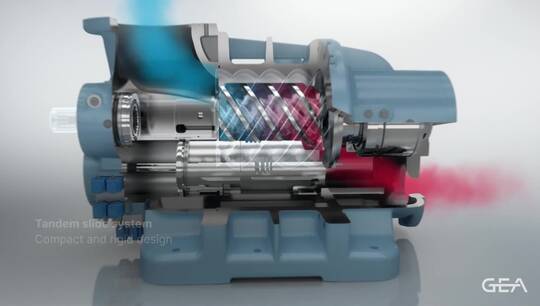
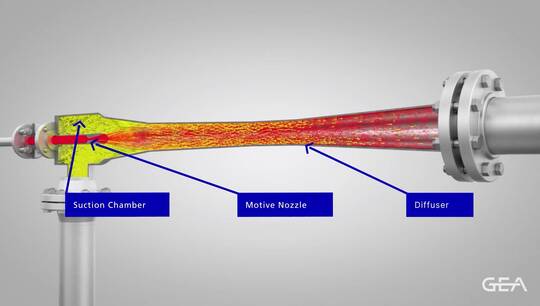
Crystallization is a single step separation process where the target chemical becomes unique from all others in solution. The result is a crystal containing only the target product which, when separated from the remaining liquid, can reach ultra-high purities (more than 99.999wt%). A system that combines distillation and crystallization can often reduce operational costs and improve purity and recovery.